On September 1, 2025, the EU REACH Amendment Regulation (EU) 2025/1731 came into effect, marking the full implementation of the new restrictions on 16 CMR substances (carcinogenic, mutagenic and reproductive toxic substances) in Articles 28, 29 and 30 of Annex XVII of REACH Regulation. This regulation update is to adapt to the classification adjustment of EU CLP regulation (namely, Regulation (EC) No.1272/2008 on Classification, Labelling and Packaging of Chemicals).
Previously, these 16 substances have been newly classified as Class 1B CMR substances in the CLP regulations of the European Union. According to the relevant provisions in Annex XVII of REACH regulations, once the substances are classified as CMR 1A or 1B, they will be prohibited from being put on the market or used for supplying to the general public (except in special circumstances) to ensure public health and environmental safety.
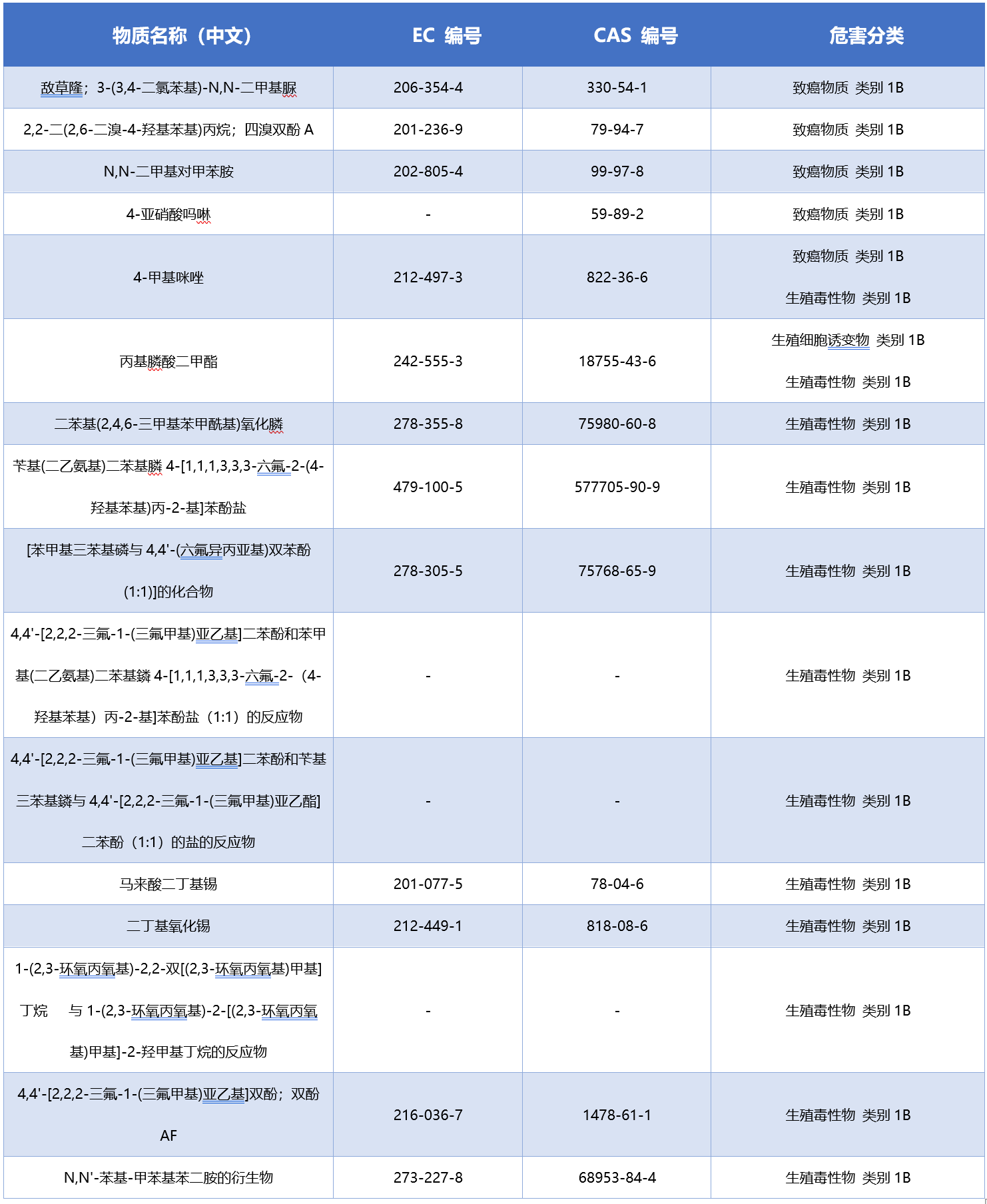
The list of 16 new CMR substances is as follows:
Suggestions on enterprise compliance
1. Screening materials: immediately check the product BOM and raw material composition against the 16 CMR material lists (including Chinese names and EC/CAS numbers) in the document; Ask the supplier for Safety Data Sheet (SDS) to confirm whether it contains such substances.
2. Concentration measurement: For the materials/products suspected to contain restricted substances, immediately entrust a testing organization with the qualification recognized by the European Union for testing, and make sure whether the actual concentration meets the requirements of laws and regulations.
3. Internal training: organize purchasing and quality control departments to study EU regulation (EU) 2025/1731, understand the restrictions and exemption conditions of the regulation, and ensure that the products meet the latest requirements.
4. Solve the problem of supply chain and formula: require suppliers to provide compliance certificates, and immediately negotiate the replacement of non-compliant materials. If suppliers cannot replace them, terminate the procurement of the materials and re-select qualified suppliers. If it is necessary to adjust the product formula, after the new formula is determined, it is necessary to entrust testing again to verify whether it still contains restricted substances.
Warm tips
With the continuous updating of Appendix XVII of REACH regulations, enterprises are faced with more and more control requirements. ZRLK suggests relevant enterprises to improve their risk awareness of products, pay close attention to the update trends of REACH regulations in time, adjust production strategies, ensure that products containing restricted substances are exported to meet the latest regulatory requirements and avoid trade risks. Our company has a professional technical team and rich experience in product testing, which can help you easily understand whether the products are safe and compliant. If you need it, please feel free to contact us, and our engineers will serve you at the first time!


![[Holiday Notice] ZRLK 2026 Chinese New Year Holiday Schedule](/uploads/image/202602/698559be66d97.jpg)










