On June 20, 2022, the European Chemicals Agency (ECHA) proposed to add hydrogenated terphenyls (CAS No. 61788-32-7) to the EU REACH Regulation (EC) No 1907/2006 Annex XVII restricted substances list for restriction, and conduct public consultations. In addition to exemptions, it is proposed to prohibit the placing on the market of hydrogenated terphenyl substances, and to prohibit the placing on the market of substances, mixtures and articles with a concentration of hydrogenated terphenyl substances equal to or exceeding 0.1% by weight.
Main uses of hydrogenated terphenyls
Hydrogenated terphenyl is a heat transfer oil with excellent performance. Its main component is a mixture of partially hydrogenated terphenyl isomers. It is obtained by partial hydrogenation of a mixture of ortho-, meta- and para-terphenyl in different proportions (saturation is 39%). .
Hydrogenated terphenyl has excellent thermal stability, anti-oxidation and low vapor pressure and other properties, it is widely used in petrochemical, synthetic fiber, synthetic resin, medicine, printing and dyeing and other industries, high temperature heat conduction, heating equipment system, chemical fiber polymerization (polyester, Nylon polymerization, dry spandex spinning), organic silicon monomer synthesis, trichlorosilane and polysilicon, pharmaceuticals, pesticides, dye intermediates and other fine chemicals, biodiesel. This substance is classified as Very Persistent and Very Bioaccumulative (vPvB) and is placed on the Substances of Very High Concern (SVHC) list (Candidates for Authorization).
Proposed restrictions:
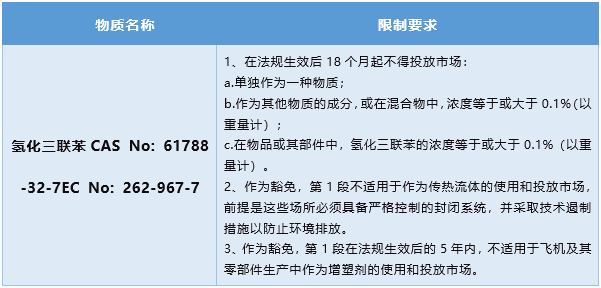
A public consultation on this restriction began on June 20, 2022 and will end on December 20, 2022. Final comments from the Risk Assessment Committee (RAC) and Socio-Economic Assessment Committee (SEAC) are scheduled to be released by the end of July 2023. ECHA will submit the joint comments of the two committees to the European Commission, which will make the final decision on whether to add the proposed restrictions to the REACH Regulations Annex XVII restricted substances list.
Reminder
With the continuous updating of REACH regulations, enterprises are faced with more and more control requirements. ZRLK recommends relevant companies to improve their risk awareness of their products, pay attention to the updates of REACH regulations in a timely manner, adjust production strategies, and ensure that export products containing restricted substances comply with the latest regulations Control requirements and avoid trade risks!