On June 3, 2025, the European Commission issued REACH Amendment Regulation (EU) 2025/1090, adding the control of item 80 N,N-dimethylacetamide (DMAC) and item 81 1-ethylpyrrolidin-2-one (NEP) to REACH Annex XVII. The revised regulation will take effect 20 days after the release.

The information of the newly controlled substances is as follows:
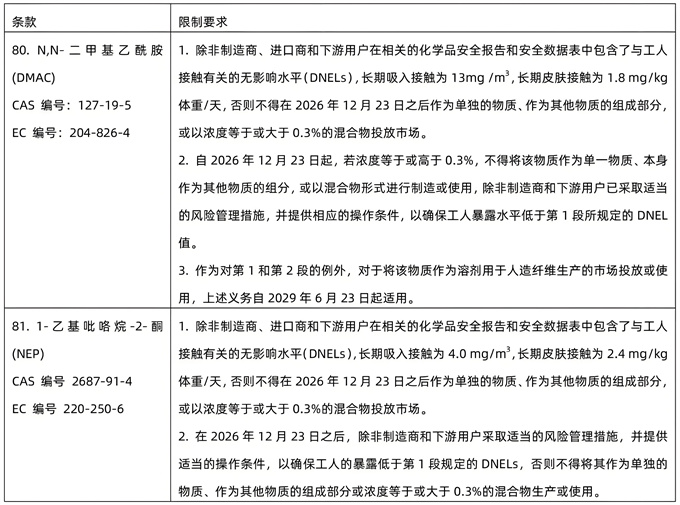
Warm Tips
With the continuous update of Annex XVII of the REACH Regulation, enterprises are facing more and more control requirements. ZRLK recommends that relevant enterprises improve their product risk awareness, pay attention to the update of REACH regulations in a timely manner, adjust production strategies, ensure that the exported products containing restricted substances meet the latest regulatory control requirements, and avoid trade risks. Our company has a professional technical team and rich product testing experience, which can help you easily understand whether the product is safe and compliant. If you need it, please feel free to contact us, our engineers will serve you as soon as possible!


![[Holiday Notice] ZRLK 2026 Chinese New Year Holiday Schedule](/uploads/image/202602/698559be66d97.jpg)










