On April 12, 2023, the European Chemical Administration (ECHA) recommended that the European Commission include eight substances, including lead, in the REACH authorization list. Once a substance is added to the list, the company will need to apply for authorization to continue using these substances.
The 8 substances to be included in the authorized substance list are:
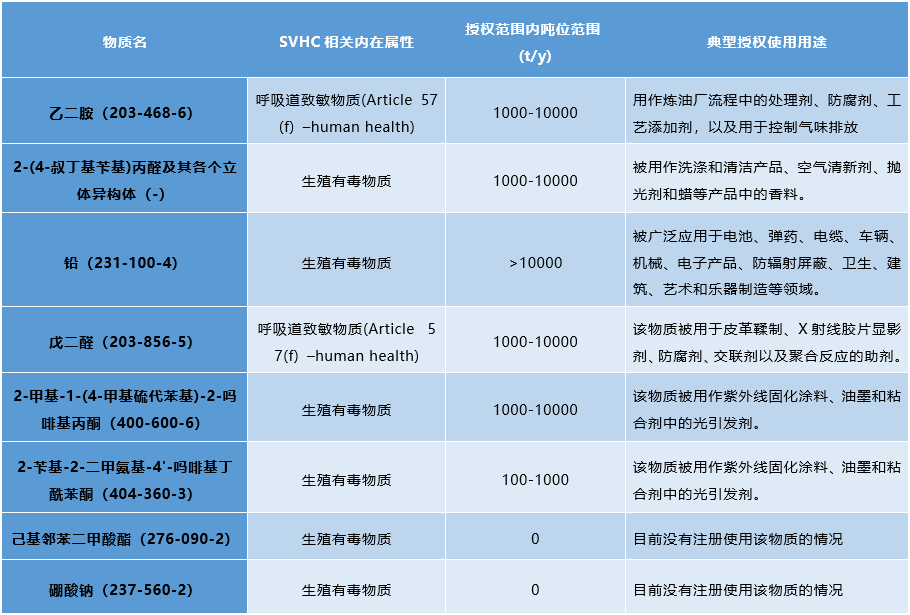
ECHA has a legal obligation to regularly recommend substances from the candidate list to the committee for inclusion in the authorized list. Before submitting recommendations to the European Commission, ECHA will take into account the opinions received during the three-month consultation period, as well as the opinions of member state committees. The European Commission will decide which substances are included in the authorization list and the conditions applicable to each substance. If a substance is included in the authorization list, it can only be placed on the European Economic Area market or used after a specified date if its specific use is authorized.
Kind reminder
Once the official authorization list is officially released, it will provide the sunset date and the latest application date. ZRLK suggests that relevant enterprises pay attention to changes in the authorization list in a timely manner. If the product involves substances included in the authorization list, they should adjust their formula in a timely manner, do a good job in authorization application, ensure the safety of the product, and reduce unnecessary trade losses. Our company has a professional technical team and rich product testing experience, which can help you easily understand whether the product is safe and compliant. If you need it, please feel free to contact us, and our engineers will be at your service as soon as possible!


![[Holiday Notice] ZRLK 2026 Chinese New Year Holiday Schedule](/uploads/image/202602/698559be66d97.jpg)










